Judge Grants Injunction Blocking MFN RuleDec. 29, 2020 The LUGPA Executive Committee authorized full support of the legal challenges to the MFN IFR, and the LUGPA Health Policy and Political Affairs apparatus was fully engaged with the plaintiffs in one of the four lawsuits challenging the MFN rule and had prepared an Affidavit of Harm to be filed if needed in support of one of the cases. LUGPA will continue to monitor the situation closely and will support legislative, regulatory and legal efforts to ensure that our member practices continue to be able to provide critically needed medications in the office setting. See also: On Nov. 24, LUGPA released it's Statement on Most Favored Nation Drug Policy. LUGPA strongly opposes the interim final rule, which would impose a “Most Favored Nation” model because it will undermine the doctor-patient relationship and threaten patient access to cancer care.
LUGPA is continuing its advocacy in collaboration with the Community Oncology Alliance (COA) which also filed a suit asking for a declaratory judgment and injunctive relief to prevent CMS’ efforts to implement the “Interim Final Rule.” LUGPA will be filing an affidavit of harm to accompany the COA suit. While the outcome of these suits remains undetermined, the temporary restraining order does delay the implementation of the IFR while the court considers pending preliminary injunctions. We will continue to closely follow the litigation as well as work with aligned stakeholders in opposition to implementation of the MFN Interim Final Rule. Further information will be provided as it becomes available.
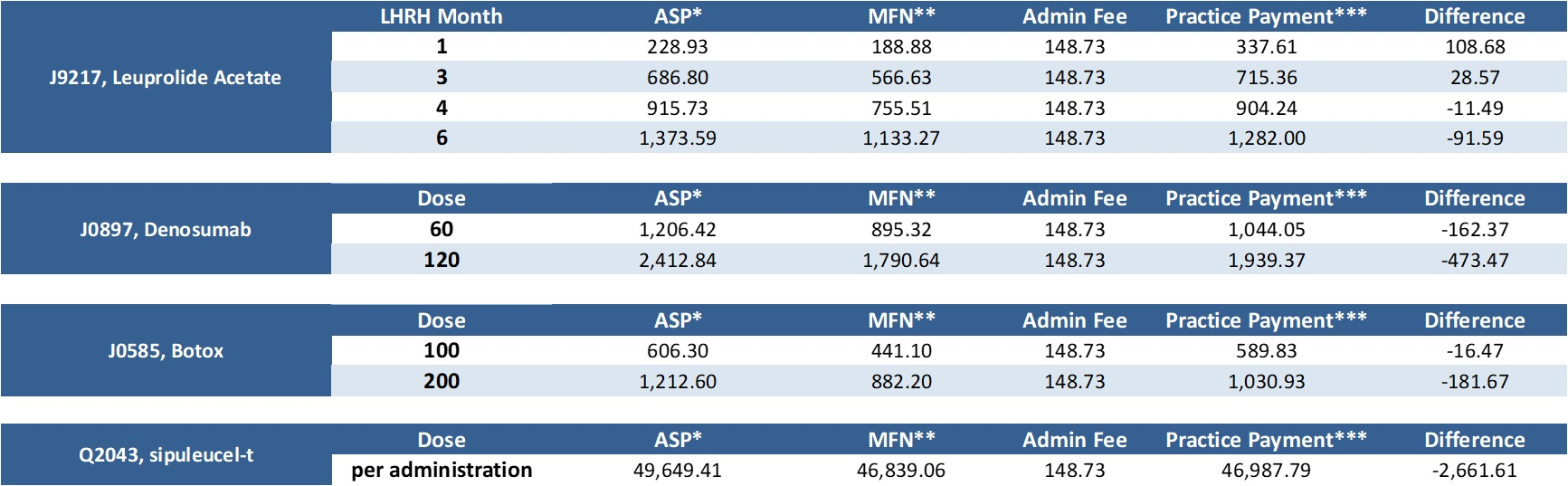
Dec. 17, 2020: CMS releases MFN Part B payment list for Q1 2021 Earlier today, CMS released the MFN Part B payment list for Q1 2021; CMS had previously released the Q1 2020 ASP list. Below represents an analysis of the proposed payments commencing January 1 for the four most commonly prescribed Part B drugs by urologists that are subject to MFN pricing. The difference represents differential between posted Q1 2021 ASP price and MFN pricing:
Click here to enlarge the chart. * Represents Medicare posted ASP inclusive of 6% add-on It is important to remember that this reflects payment data only, this does not factor in the impact of acquisition costs or any rebate programs. Also, this represents national payment data, each individual MAC can make local coverage determinations. Certain institutional providers are exempt from this program as well. While this information represents the organization's best efforts to analyze the data, this data is for informational and illustrative purposes only; LUGPA as an association does not become involved in the competitive business decisions of its individual members, nor will it take any action that would tend to restrain competition. LUGPA IS NOT ADVISING NOR ENDORSING ANY CHANGE IN CLINICAL OR BUSINESS DECISIONS BASED ON THE ABOVE DATA. LUGPA strongly encourages each member practice to perform its own analysis and in all cases, to act in the best clinical interest of its patients. LUGPA, along with a myriad of other stakeholders, is adamantly opposed to implementation of this rule. We will continue to keep you apprised of developments as they unfold. |